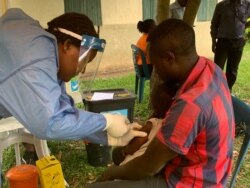
Uganda has started its largest Ebola vaccine trial to date, health authorities announced Monday, in an apparent effort to prevent the disease from spreading.
An epidemic across the border in neighboring Democratic Republic of Congo has killed over 1,800 people, making this outbreak the second-deadliest to date, with fatality rates nearing 70%.
The experimental Johnson & Johnson vaccine will be administered to health care professionals, as well as ambulance drivers, burial teams and cleaners. The trial is expected to last two years and cover 800 people in the Mbarara district in southwest Uganda.
Vaccinations have already begun, according to Uganda's Medical Research Council.
There are no licensed treatments for Ebola, but one vaccine, manufactured by Merck, was used effectively at the end of the 2013-2016 outbreak in the DRC and has been used during the current epidemic. Over 180,000 people have received this vaccine.
But the supply is sporadic, and vaccine administrators are typically 1,000 doses short of what they need, according to Doctors Without Borders as reported by Bloomberg News. Health professionals have called for the use of both the Johnson and Merck vaccines to maximize the number of people protected from Ebola.
Some people, including the DRC's former health minister, opposed the move, arguing that another vaccine with a different administration schedule would stoke vaccine distrust in vulnerable areas.
While the Merck vaccine is administered through one shot and takes 10 days to be effective, the Johnson & Johnson vaccine requires two shots, two months apart.
Aside from sparking anti-vaccine fear, the Johnson & Johnson drug could be difficult to administer in practice, as violence in northeastern DRC hampers disease-control efforts.
Neighboring countries have been on high alert since three people died of Ebola in the DRC city of Goma, located on the border with Rwanda and just a few hours from Uganda.