As the global fight against AIDS intensifies, activists have placed increasing importance on getting people to know their HIV status.
For decades, learning one’s HIV status involved going to a clinic, taking a test, and then waiting for days or even weeks for the laboratory results to come back. But simpler, faster and more private testing methods are being developed, with promising signs of success. Some were on display at the International AIDS Conference here.
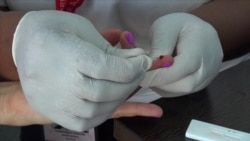
The Advanced Quality Anti-HIV Rapid Test, approved by the World Health Organization, is being used by many conference attendees.
"It is very quick and so you can pick up whether the person is HIV positive or not," said Dr. Thandeka Khoza, senior medical officer for North Star Alliance’s southern Africa region. The nongovernmental organization has used the method in targeting truck drivers and sex workers in more than 10 African countries.
"You just take the patient’s blood and put it in the machine, and in 15 to 20 minutes the person knows their CD4 count," Khoza said.
The laboratory test measures the strength of a person’s immune system, looking at the number of CD4 T lymphocytes, or CD4 cells, in a blood sample, as the AIDS.gov website explains. The white blood cells help fight infection.
Speedy results
Kits like the Western blot and Ebon can quickly analyze blood samples, meaning patients no longer have to wait for days for laboratory test results.
The new technologies accommodate patients who do not even want a doctor to know their status. The OraQuick In-Home Oral HIV Test kit, still under trial, allows people to test privately at home.
"The advantages of self-testing are that it is confidential and more convenient than facility-based testing, and allows a lot more privacy and convenience to the user," said Yilu (Lulu) Qin, a fellow with the University of North Carolina’s Institute for Global Health and Infectious Diseases. Its UNC Project-China conducted an at-home testing trial that she said was well-received.
Sensitivity stressed
Alere, a global diagnostic device and service provider, is seeking the WHO’s approval for its Alere HIV Combo. The kit purportedly is sensitive enough to detect the HIV virus in blood samples even during the first three to four weeks after exposure – the so-called window period – and in newborn infants.
If approved, the Alere HIV Combo would be administered by professionals at a health facility, as is the case with the Advanced Quality Anti-HIV Rapid Test.
Those seeking approval of the OraQuick In-Home Oral HIV Test kit hope to make it available in drugstores.