U.S. health officials said Thursday a company is recalling its over-the-counter eye drops that have been linked to an outbreak of drug-resistant infections.
The Centers for Disease Control and Prevention this week sent a health alert to doctors, saying the outbreak included at least 55 people in 12 states. One died and at least five others had permanent vision loss.
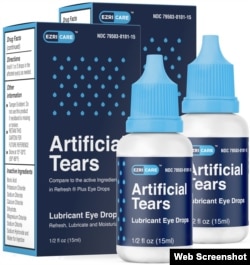
The infections, including some found in blood, urine and lungs, were linked to EzriCare Artificial Tears. Many said they had used the product, which is a lubricant used to treat irritation and dryness.
The eye drops are sold under the name EzriCare and are made in India by Global Pharma Healthcare. The Food and Drug Administration said the company recalled unexpired lots of EzriCare Artificial Tears and another product, Delsam Pharma's Artificial Tears.
The FDA recommended the recall based on manufacturing problems including lack of testing and proper controls on packaging. The agency also blocked import into the United States.
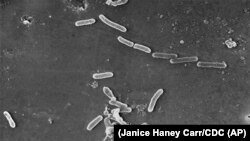
The infections were caused by a bacteria called Pseudomonas aeruginosa. Investigators detected it in open EzriCare bottles, but further testing was underway.
EzriCare, the company that markets the eye drops in the U.S., said it is not aware of any evidence definitively linking the outbreak to the product, but that it has stopped distributing the eye drops. It also has a notice on its website urging consumers to stop using the product.
Infections were diagnosed in patients in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington and Wisconsin. A person in Washington died with a blood infection.
The outbreak is considered particularly worrisome because the bacteria driving it are resistant to standard antibiotics.
Investigators found the bacteria were not susceptible to any antibiotics routinely tested at public health laboratories. However, a newer antibiotic named cefiderocol seemed to work.
How could eye drops cause infections in the blood or lungs? The eye connects to the nasal cavity through the tear ducts. Bacteria can move from the nasal cavity into the lungs. Also, bacteria in these parts of the body can seed infections at other sites such as in the blood or wounds, CDC officials said.