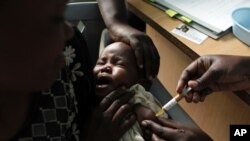
The world's first experimental malaria vaccine produced disappointing results in a large-scale test among African infants, raising questions about its potential for fighting the disease.
The vaccine, promoted as a new weapon in the malaria fight, reduced the risk of malaria by only 30 percent. The study involved more than 6,500 babies aged six to 12 weeks.
The results, released Friday, showed the vaccine providing less than half the protection it did in a previous smaller trial involving infants. The report said the "modest protection" the vaccine, which is also known as RTS,S or Mosquirix, has been provided in this latest trial was also lower than the 50 percent reported last year among older children.
Dr. Jennifer Cohn, a doctor with Doctors Without Borders, told the Associated Press that the vaccine’s effectiveness was “unacceptably low.”
Vaccinating babies is seen as a more cost effective way of battling the disease since it could be added to the regimen of other infant vaccinations.
Billionaire Microsoft founder and philanthropist Bill Gates, whose foundation is helping fund the vaccine, said the effectiveness rate came back lower than hoped.
But the top British drug manufacturer developing the vaccine, GlaxoSmithKline (GSK), will continue its efforts. Chief executive Andrew Witty said the drugmaker remains convinced the vaccine has a role to play in tackling malaria.
“We’ve been at this for 30 years, and we’re certainly not going to give up now, he said during a conference call with reporters.
The company, which has invested $300 million in the drug, does not expect to profit from the drug, which will be sold only in poor countries.
“The results look bad now, but they will probably be worse later,” said Adrian Hill of Oxford University to the Associated Press.
The results were released during a conference in South Africa Friday as part of a continuing study that will end in 2014.
The World Health Organization estimates that more than 650,000 people die from the mosquito-borne illness each year. The vast majority are children in sub-Saharan Africa.
The vaccine, promoted as a new weapon in the malaria fight, reduced the risk of malaria by only 30 percent. The study involved more than 6,500 babies aged six to 12 weeks.
The results, released Friday, showed the vaccine providing less than half the protection it did in a previous smaller trial involving infants. The report said the "modest protection" the vaccine, which is also known as RTS,S or Mosquirix, has been provided in this latest trial was also lower than the 50 percent reported last year among older children.
Dr. Jennifer Cohn, a doctor with Doctors Without Borders, told the Associated Press that the vaccine’s effectiveness was “unacceptably low.”
Vaccinating babies is seen as a more cost effective way of battling the disease since it could be added to the regimen of other infant vaccinations.
Billionaire Microsoft founder and philanthropist Bill Gates, whose foundation is helping fund the vaccine, said the effectiveness rate came back lower than hoped.
But the top British drug manufacturer developing the vaccine, GlaxoSmithKline (GSK), will continue its efforts. Chief executive Andrew Witty said the drugmaker remains convinced the vaccine has a role to play in tackling malaria.
“We’ve been at this for 30 years, and we’re certainly not going to give up now, he said during a conference call with reporters.
The company, which has invested $300 million in the drug, does not expect to profit from the drug, which will be sold only in poor countries.
“The results look bad now, but they will probably be worse later,” said Adrian Hill of Oxford University to the Associated Press.
The results were released during a conference in South Africa Friday as part of a continuing study that will end in 2014.
The World Health Organization estimates that more than 650,000 people die from the mosquito-borne illness each year. The vast majority are children in sub-Saharan Africa.