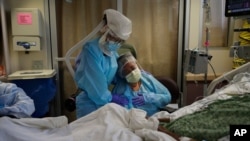
Almost 1,400 frontline health care workers in the U.S. have apparently died of COVID-19, according to a joint investigation by British newspaper, The Guardian, and Kaiser Health News. One-third of the dead health care workers were nurses, the study said.
Many of the health care workers, the report said, “are struggling with illness, trauma and exhaustion.”
A surgical nurse told The Guardian that in the first two months of the coronavirus pandemic, he wrapped more people in body bags than he had in the previous 25 years of his career. Jim Gentile said, “Many of us have PTSD.”
More than 58 million people around the world have been infected with the coronavirus, the Johns Hopkins Resource Center reported early Sunday.
The U.S. continues to lead the world in infections with more than 12 million cases, followed by India with 9 million infections and Brazil with 6 million.
The virus has claimed more than 1.3 million lives worldwide. More than a quarter million of those deaths were in the U.S.
Even though the coronavirus disease is surging, not everyone is eager to be vaccinated against it, according to a recent Ipsos poll. While 73% of those polled worldwide said they would be vaccinated, that number was four points higher this summer.
The study found that “vaccination intent” declined in 10 of the 15 countries included in the poll. Intent went down the most in China, Australia, Spain and Brazil.
The U.S. Food and Drug Administration Saturday authorized the emergency use of a COVID-19 antibody therapy that President Donald Trump said helped cure him of the disease caused by the coronavirus.
The Regeneron Pharmaceuticals Inc. therapy approved by the FDA is made up of the monoclonal antibodies, casirivimab and imdevimab. They are to be administered together to treat mild to moderate COVID-19 in adults, including those 65 and older with some chronic medical conditions, and children who are at high risk of a more severe case.
The company expects to have enough of the treatment ready for about 200,000 patients by the first week of January.
Friday, U.S. pharmaceutical company Pfizer and its German partner, BioNTech, said they have filed for emergency authorization from the U.S. Food and Drug Administration to use their COVID-19 vaccine, saying they are poised to begin distribution within hours of receiving approval.
The application comes after the companies said testing shows the vaccine has an effectiveness rate of 95%, with no serious safety concerns observed to date.
U.S. Health and Human Services Secretary Alex Azar said Friday that the FDA could decide about emergency use for the vaccine candidate within weeks.